Anthia Maysidue is from the Department of Chemical Engineering and Biotechnology and a member of the BioElectronic System Technology Group. Here, we are sharing her virtual lecture for Cambridge Festival 2021, on ‘Organs-on-Chips.’ Visit here.
To do that, the gut is equipped with a layer of several types of epithelial cells that form a selective barrier between the gut lumen and the bloodstream. So, we identify this as the functional unit of our organ-on-chip. Taking into account the tubular architecture, we developed the 3D organ-on-the-chip and named it L-Tubistor. We also pottered a hollow channel in between to mimic the lumen of the gut. There are also two gold electrodes around the polymer scaffold. These help us establish contact with the material to measure the electrical properties at any given time. We also use fibroblasts to mimic the interface between the gut barrier and the blood vessels. After almost a month, we see the cells forming a structure very close to the one in our body.
The polymer used is an electroactive conductor. An electron inside the system helps to see how cells proliferate and work inside the tissue. By evaluating these electrical read-outs, we can characterize the integrity of the intestinal barrier. This integrity is a critical parameter for a healthy, tight, and functional intestinal barrier. This barrier targets some pathogens and toxins, and inflammation means that the barrier is significantly damaged. We now have data to show that we can use our bioelectronic chip to show depth to model situations like inflammation and test other potential drugs to see how they travel these barriers in the bloodstream and eventually reach the target organ.
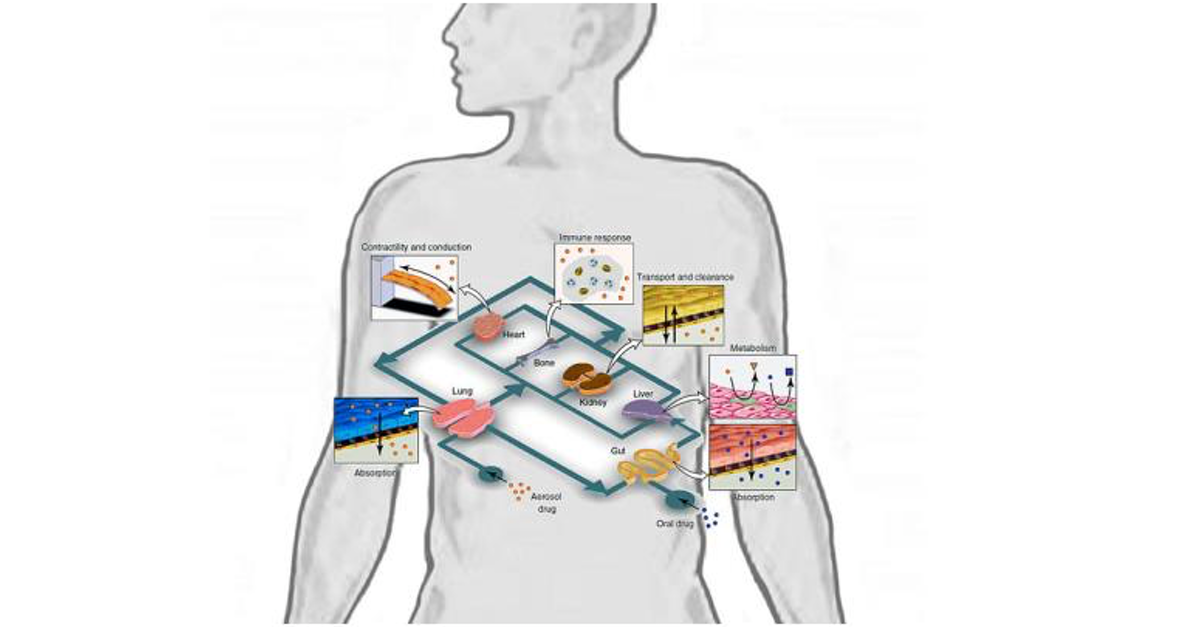
While organ-on-chips are of great use for both organ level and fundamental cell biology studies such as pharmaceutical testing, the greatest potential of this technology is revealed by connecting multiple organs-on-chips in one platform. We are going now towards multi organs-on-chips. Such platforms would allow organs to grow independently and accurately mimic the function of the human organ. At the same time, the organs can interact with each other, simulating how they interact in the body.
Currently, there are two approaches for building multi organs-on-chips. The first is by linking organ-on-chips together via their fluidic channels. The second is growing different organs in the same chip and connecting them with different microfluidic channels but in one platform. Now, why is this useful, and how can it benefit drug testing? By joining together different organs in one single network, we can follow a drug through its journey to its target through a simulated human body.

Let’s take the example of a drug taken by an asthma inhaler. In a multi organ-on-chip platform, we could monitor in real-time how the drug enters the lungs and affects the heart, how it is metabolized in the liver, how the kidneys excrete it, and if there are any side effects along this journey.
Let us see some applications. One example where living animals must be used in preclinical trials is the characterization of a drug’s pharmaceutical kinetics. It refers to the quantification of drug absorption, metabolism, and excretion. And all these data help us determine the effects the drug produces on the target organ, which helps us see the mechanism of the drug and identify and measure its adverse effects. All these responses involve interactions between different organs in our body and the bloodstream. Or in other words, we need the whole body’s response to determine this pharmacokinetics and pharmacodynamics.
Until now, animal models were the only way we could carry out such researches. In an example of a multi-human organ-on-chip system, the research team created a functional platform to obtain experimental data. Together with computational modeling, they were able to generate data about all pharmacokinetic and pharmacodynamic behavior of drugs in humans.
So, firstly what they did was add nicotine drugs. This is used as an oral drug for neurodegenerative disease. They added it to the gut chip in the epithelial channel to mimic the oral administration of the drug and also the absorption into the bloodstream in the intestinal wall, how it gets into the liver and how it goes into the liver and gets metabolized and how it goes to the kidney where it is excreted.
The researchers also took samples; in the same way, we go to a clinic to get a blood test, which helped them and the computational modeling to translate the data from the organ chips to the actual organ in the human body. They saw that the platform and their experiments were matching in their findings. So this is an excellent sign that the results were matching in the human trials, and the organ-on-chips can be implemented in clinical trials in drug testing. They also tested cis-platin, a chemotherapeutic drug commonly used in cancer treatments. This one is administered intravenously, but here we have bone marrow, liver and kidney chip. The experiment also produced information about how this cis-platin is metabolized and created by these organs, and they produced results similar to human body responses. So, we can get accurate pre-clinical testing of drugs and viable information about the human body outside the human body and without animal models.
Recently, another multi-organ chip was developed which was able to link up to ten different organs. It’s called Physiome-on-a-chip. Although the tissues don’t replicate into an entire organ, they can perform many of their functions. Each organ model can be scaled-up or scaled-down and can tune the operation principles to accommodate its application requirement. The researchers used this system to show that they could deliver a drug to the gut mimicking oral administration. They then observed the drug as it transported to other parts, metabolized, etc., and they would also measure where the drugs went, which organ chips in this platform, which affects these different platforms, and how the drug was broken down. This kind of system holds great potential for accurate prediction of complex adverse drug reactions on the human system, so I think this is a new approach in drug delivery, and we can see this happening.
In our group, we are working on multi-organ systems, in particular, we are trying to build a platform using the gut-brain-axis-on-a-chip to mimic the way the gut communicates with the brain. We want to use this platform to provide the researchers with the tools to test candidate drugs for intestinal and neurodegenerative diseases. But what we are really interested to study in is how the gut microbiota, the community of microorganisms that resides in our gut affect our health, how they are involved in diseases of organs, particularly the brain, and we hope we’re going to use this 3D organ-on-a-chip as our lego brick to build our platform.
In summary, I hope I convinced you that organ-on-a-chip technology is a better way to mimic human biology. Until now, we could examine cells in a dish and look at patients in a clinic, but there was a missing link in between. These chips have risen as the bridge between these two and have opened a new window into human biology for us. The chip allows us to see biological activities in human cells and tissues and activities unique to human cells and not animal cells. This is particularly important in drug delivery. We can test drug candidates with this chip to counter the ‘death valley’ discussed initially. Drugs can decide to move to human trials and see their efficacy in humans.
There are quite a few pharmaceutical companies using organ-on-chips coming out of university research across the world, including Cambridge University, and it has been shown that we get completely different results between animals and chips. Most people forget that we don’t actually predict what happens in humans while using animal models. This technology offers a solution to this. The more people become familiar with this, the more chances of faster drug delivery and lower costs.
The next level of organ-on-chips involves finding medicines for humans; they can use them to find the best medicine for each person. So, ‘Your body-on-a-chip’ is the new thing. People’s bodies react differently to medicines, the dosage required, etc. In the future, we might go to painlessly donate cells to create personalized medicines with our body-on-a-chip, a model that will represent individuals and test whether a specific medicine will work for us.
Read Part One And Two here:

Aniqa Mazhar is a graduate of QAU in Biochemistry. She has taught sciences to O levels and is currently planning for her MS in Food Technology. Aniqa’s hobbies are reading, watching movies, writing, calligraphy, long walks, and nature photography.