The hole in the ozone layer that develops annually is “rather larger than usual” and is currently bigger than Antarctica, say the scientists who have monitored it for decades.
Researchers from the Copernicus Atmosphere Monitoring Service say that this year’s hole is overgrowing and is more significant than 75% of ozone holes at this stage in the season since 1979.
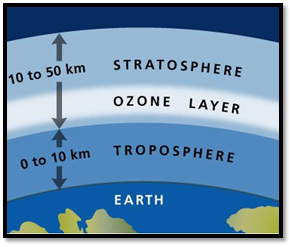
The ozone layer. Ozone cover, a safe zone for Earth, is one of the predominant features supporting life on this planet. Engulfing the upper layer of the atmosphere, the stratosphere (between 10 km to 50 km altitude), ozone comprises three oxygen atoms (O3).
This chemical composition of the layer makes it reactive towards different other chemicals. This blanket prevents ultraviolet rays of a wavelength less than 290 nm (nanometer) from reaching the Earth by absorbing them. Not all the ozone resides in the stratosphere (the upper atmosphere): a part of it lies in the troposphere, the lowest region of the Earth’s atmosphere. Ozone depletion is a matter of grave concern and for all the right reasons.
Meeting Ozone
Ozone gas (O3) is a pale blue gas with a pungent smell composed of inorganic molecules. The ozone layer is thicker in the atmosphere over the poles as compared to above equator. It functions by absorbing the ultraviolet rays (UV) of the highest energy, the UV-B and UV-C.
How is it produced?
The concept of a white blanket surrounding the Earth seems mythological; its presence is what has made the Earth a habitable planet for all. The phenomenon of its production is a crucial aspect in understanding its role. The oxygen molecules in the atmosphere are split into individual oxygen atoms due to the action of the sun’s ultraviolet rays. These single oxygen atoms have the affinity to bind with adjacent oxygen molecules to form ozone (O3). Ozone is the result of both natural processes and human activities.

Ozone as sanctuary
Ozone is a protective duvet around the Earth that absorbs the sun’s harmful ultraviolet rays, thus halting them from reaching the Earth’s surface. In the absence of ozone, Ultraviolet rays would reach the surface and cause severe damage that includes skin cancers, eye cataracts, and damaged DNA in plants and animals, leading to various mutations. Scorching heat and high energy of Ultraviolet rays negatively affect plant and plankton growth. Plankton or marine drifters form the base of the food chains, responsible for maintaining the food chain. Their lack of production will disturb the majority of food chains and hence be detrimental to the survival of other organisms.
Commination to Ozone
Prospective scarring of ozone became an eyeopener when a Dutch scientist, Paul Crutzen, published a paper showing that the nitrogen oxide catalytic cycle negatively affects ozone layer concentration. In the 1970s, the production of supersonic transports (SST) was resisted due to their potential release of nitrogen compounds, primarily nitrogen oxides, which could impair ozone. This phase marked the enlightenment of the scientific society with the realization that the ozone layer was diminishing.
The concept of a white blanket surrounding the Earth seems mythological; its presence is what has made the Earth a habitable planet for all.
On a larger scale, there are two significant reasons for ozone depletion: Anthropogenic and Natural.
1. Anthropogenic activities
Commonly known as human activities, anthropogenic activities increase the concentration of atmospheric pollutants, thus playing a significant role in ozone slumping. Chemical compounds (Ozone-Depleting Substances) that release gaseous chlorine and bromine are most prone to ozone damage. Ozone-depleting substances (ODS) that release chlorine encompass chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), carbon tetrachloride, and methyl chloroform. Once CFCs are released into the atmosphere, they make their way to the upper layers of the atmosphere stratosphere, where the action of ultraviolet rays breaks them. This dissociation liberates chlorine atoms which are detrimental to ozone.
Halogens and methyl bromide are the ODS that liberate bromine. These chemicals are released on the Earth’s surface; they can travel to the stratosphere, thus posing a threat to the ozone layer.
Another drawback of ODS is that they remain within the atmosphere and do not come down to land with rain. Thus, their ability to stay within the atmosphere for a more extended period makes them harmful substances to the ozone layer.
Key ODS sources
- Halocarbon refrigerants
- Aerosol propellants
- Fire extinguishers
- Foam blowing agents

2. Natural causes
Sunspots, volcanic eruptions, and stratospheric episodes pose a small percentage (1%-2%) of threat to ozone depletion.
The Current Status
The ozone hole over the Antarctic is the biggest one till now. Till early October 2020, it covered an area of 24 million square kilometers. Post extensive studies and close monitoring of the ozone hole, the scientists at NASA claim that the ozone hole over the Antarctic has reached its peak. According to the UN Environment Programme Scientific Assessment of Ozone Depletion (presented in 2018), it is stated that the ozone layer will possibly recover to its pre-1980 levels by 2060. However, this ozone depletion is not restricted to the Antarctic only but occurs over other regions, including North America, Europe, and Asia.
The Unfortunate Effects
Ozone shrinking is a matter of grave concern giving rise to numerous unfavorable aftereffects. Some of the significant drawbacks of ozone depletion include:
- Damage to human health
Exposure to high-energy ultraviolet rays can cause cancers, severe sunburns, and the gradual weakening of the immune system. Such adverse effects can subside the quality of life.
- Environmental deterioration
Plants also have a range of tolerance of UV light of a given energy. Continuous exposure to highly high-energy UV rays can hamper the average growth and reproduction of plants, such as the process of germination, photosynthesis, and flowering. Crops sensitive to high-energy UV radiation include wheat, barley, tomato, etc.
- Risk to Marine life
Just like land beings, life underwater is also exposed to energetic UV radiations. These high-intensity radiations can prove to be detrimental to plankton, the basis of the food chain. If plankton growth is reduced, it will disrupt the entire food chain mechanism and thus the productivity of sea life.
Ozone for life
Restoring ozone levels to how they initially were, is a vast but acute undertaking. The Montreal Protocol of 1986, an international agreement aimed to address the ozone depletion issue, should be focused on. Firstly, we need to cut down cleaning agents containing corrosive and harmful solvents and replace them with non-toxic agents. Secondly, there is a need to minimize the use of ample transport vehicles. Rather than using a separate transport accommodation, where possible, we should use public transport.
This will contribute to a decrease in the carbon footprint. Thirdly, to further narrow down the carbon footprint, we must source and utilize locally manufactured products. In addition, proper maintenance of refrigerators and air conditioners should be done as their inefficient working can release CFCs into the air. Though the recovery process is time taking, one step at a time can make a crucial difference.
Earth without ozone is like a house without a roof.
Also, Read: Heal the Earth, Heal yourself

Maira Masood is a BS Biosciences student at NUST, Pakistan. She aspires to be a geneticist and wants to play an active part in spreading scientific awareness through writings.

