We all acquire infections throughout our lives, right from the neonatal period to late old age. We go through the natural history of infections that being from incubation periods to the development of immunity and resolution. While the common person is well aware of the fact that we gain resistance against infections by actually suffering from them initially, little does the common folk realize that while our bodies’ defense systems protect us in the long run, it may also prove to be the proverbial double-edged sword.
Before we delve into the perspective behind the preset set earlier, it’s pertinent to mention the burden of chronic disease in our current day and age. Chronic and especially cardiometabolic diseases (which comprise the lion’s share ) are the culmination of multiple risk factors ranging from genetic predispositions and sedentary lifestyles to stress. However, there is one binding factor behind most, if not all, chronic diseases, and that is chronic inflammation. Inflammation is an alarm state in the body, constant stress with heightened levels of markers of biological stress. What this can do is affect every organ system of the body.
For example, inflammation sets off in the fatty tissue and pancreas, leading to diabetes mellitus, or in the vasculature of the body leading to heart disease or even strokes. Mind it; inflammation is in no way a ticket to the land of doom and gloom. Inflammation Is an intricate process set off in our bodies in the face of infections or any other insult. This is an innate response of our immune system and vital to our survival. It is this very response when continuing unabated in response to an infection, that can lead to a host of morbid conditions in the long run and even acutely in some cases.
The whole process of mounting immune responses to antigens does not happen overnight. It starts off way back when we are babies.
The beginning
When we are born, our bodies are not used to all the different types of antigens all around, and as such, we have no protection against them. As we are gradually exposed to what the world has to offer, a specific gland in the lower part of our neck called the Thymus starts producing what I would label as little ‘armies’ or ‘clones of armies’, the lymphocytes which actually will be responsible for setting up immune responses in the future against particular antigens.
Another essential process that goes on inside the gland is eliminating those ‘clones’ that target our own tissues. A process called immune tolerance to our own tissues. It is this very process when gone haywire leads to the non-exhaustive list of autoimmune diseases where the body’s own organs are targeted by the immune system.
Immune response to the invaders
As we grow older, we continuously experience infections by different pathogens, namely viruses, bacteria, fungi, and parasites. Our immune system, on being exposed to them, builds immune responses to them and, in most cases, contains them and gives our bodies arsenal in the form of alert lymphocytes and other early response cells, which will immediately target the offending agent and contain it and the next time it makes an intrusion, contain it. The immune response which is built when we are exposed to infectious agents can be experienced by us as fever, the feeling of being unwell, fluctuations in pulse rate and blood pressure, and other organ-specific symptoms according to the organism involved.
Genes as determinants of the immune response against pathogens
As discussed previously, the body mounts responses to every kind of antigen we are exposed to since childhood, and most of the time, we are protected against those antigens throughout life. The process of recognition of antigens in organisms is driven by genes that determine the presence of specific proteins called the ‘major histocompatibility complex (MHC)’ in the immune and basically every cell of the body. These proteins are where the antigens of invading pathogens attach and the whole cascade of immune containment and inflammation begins. In humans, the genes for the synthesis of MHC are found on chromosome 6 of the human genome1.
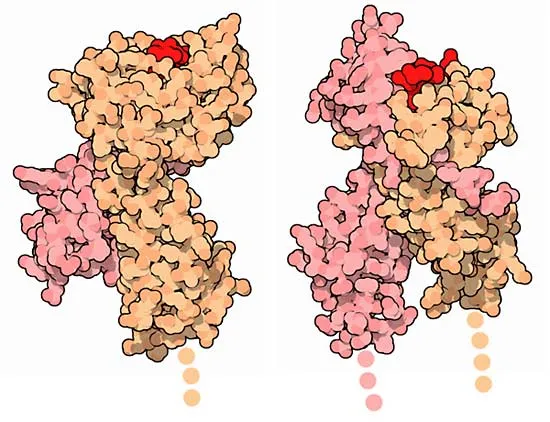
The pathogens attach to the MHC and are then presented to specific immune cells called T-Lymphocytes. These cells are the master controllers of the whole immune response. There are countless other receptors on different cells of different body organ systems by which pathogens enter cells and cause inflammation. So, one can fathom how our immunity and reaction to pathogens is dependent upon our genome.
Pathogens causing chronic diseases: The basis of it
As elaborately detailed earlier, the body’s response to infections may eventually be the harbinger of morbidity and disabilities in the long run. Medical literature abounds with associations between pathogens and chronic illness and even cancers, though there is no definitive cause-and-effect relationship in most cases. Strong relationships exist between many organisms with cancers and serious illnesses.
Helicobacter Pylori and its association with gastric cancers, borrelia and its association with a constellation of symptoms of Lyme’s disease, Hepatitis B and C viruses as a cause of chronic hepatitis and multi-organ involvement, and liver cancer, Epstein Bar virus and its association with Burkitt’s Lymphoma and cancers of the throat2. Besides, numerous chronic diseases have been postulated to have etiological links with infections, e.g., type 1 and 2 diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus (SLE), and chronic ischemic heart disease. This list is non-exhaustive.
Why the above associations Have popped up over the last few decades? The answer is multifactorial firstly because we have seen a surge in the appearance and detection of zoonotic(infections transmitted from animals to humans) infections. Secondly, newer laboratory diagnostics like Polymerase chain reaction (PCR) and other advanced immunological techniques. Thirdly, Environmental factors which have gone from bad to worse in terms of high-risk behaviors, pro-inflammatory diets and mental stress all contribute in putting our immune systems into overdrive. Plus, it’s never 1+1 in disease causality. Individual genetic variations eventually determine how a particular individual will be affected acutely and in the long run by a particular pathogen.
Multiple sclerosis(MS) is one of the most prevalent neurodegenerative disorders in the world. It affects the central nervous system, i.e., the brain and spinal cord, and presents a constellation of symptoms, including motor, sensory, ocular, bladder, and bowel problems.
Disease examples
While Type 2 Diabetes Mellitus(T2DM) is a ubiquitous disease due to its solid genetic origins and environmental triggers, the etiology of Type 1 Diabetes Mellitus is a less frequent disease but with origins that are rooted in genetic inheritance only partially. Associations have been found between human enteroviruses (HEV), Rotaviruses, Cytomegalovirus (CMV), and Mumps virus, to name a few3, 4,5, which mainly target the insulin-producing Beta cells in the pancreas causing inflammation against self-tissues (autoimmunity) and, tissue destruction and eventually Diabetes Mellitus which is insulin dependent.
But on the contrary, as per the ‘ultra-clean hypothesis’, children who are less exposed to infections due to enteroviruses during early childhood are more susceptible to infections by diabetogenic viruses as they grow older. This probably relates to the paucity of protective antibodies to these viruses due to the lack of milder frequent exposure during childhood6.
However, clear-cut causality has not been established either way, but what is known is that viruses attach and enter the pancreatic beta cells through cell surface receptors which are proteins7. These proteins are synthesized from genes, and inflammatory complications start here when the viruses attach. There then is local damage to the beta cells and the damage caused by the immune cells to which the viruses attach and trigger inflammatory response8 cascades, akin to wildfire, more begets more.
Cardiovascular disease (CVD) is a scourge of the modern world, from genetics to hypercholesterolemia to Diabetes. Numerous factors play their part in its genesis9. However, the role of chronic inflammation in initiating or precipitating the worsening of CVD cannot be discounted10. CVD is not just a disease localized to the vasculature of the heart. It is the end process of what goes in the whole body’s vessels. Numerous pathogens have been implicated, namely Hepatitis C virus (HCV), Human immunodeficiency virus (HIV), Chlamydia pneumonia, and Herpes Simplex virus (HSV), to name a few.
The process of the dreaded blockage of coronary or peripheral vessels starts when there is plaque formation ( accumulation of dead immune cells, platelets, and cholesterol) and rupture. The organisms mentioned earlier have been found in plaques in the vessels’ inner linings. They have been found to enter through specific adhesion molecules on the lining surface, which these pathogens induce. These molecules then facilitate the entry of pathogens, and the process of accumulation of dead immune cells, cholesterol, and platelets starts, eventually causing rupture of the plaque, which is the harbinger of ‘heart attacks11.
Multiple sclerosis(MS) is one of the most prevalent neurodegenerative disorders in the world. It affects the central nervous system, i.e., the brain and spinal cord, and presents a constellation of symptoms, including motor, sensory, ocular, bladder, and bowel problems. This disease does not have a hereditary basis though there is a genetic predisposition augmented by environmental factors and infections to manifest the disease.
Again here, like in the previous instances mentioned, associations of this disease have been found with a few infections, of which Epstein Bar virus (EBV) is worth mentioning. This virus is notorious for triggering/ causing a host of cancers like nasopharyngeal cancer and Lymphomas12. It has also been associated with autoimmune disorders. There is a pool of data from epidemiological studies, along with studies that isolate the virus from tissues, that it is implicated in causing this disease13. Though it is worth mentioning that not all EBV infections will cause disease, it is pretty ubiquitous, and most people will have antibodies to it.
It is a particular group of people genetically predisposed to developing MS in whom this virus will cause the transformations and cascades required for the disease. The vital mechanism proposed is the entry of the virus into B-cells (mainly lymphocytes concerned with antibody production), after which it takes over the genome of the infected cells and causes dysregulated immune responses. This culminates in the production of antibodies and an immune reaction towards the myelin sheath (covering of nerves and central neural tissue) plus also against the cells which are responsible for the protection of the sheath12.
This produces the myriad of symptoms of MS, which can be progressive and relentless. Here we can see again the interplay of proteins on the surface of cells that serve as targets for the virus and genes that produce those proteins.
Relevance of the gene-infection-inflammation-disease interplay and novelty in treatment
The concept of infections causing chronic diseases and their prevention with vaccination has been investigated for some time now. However, the results have not been encouraging. The probable reason behind this can be the multifactorial nature of many diseases plus the individual variation in susceptibility to infections. Our response to such a conundrum should be multi-tiered.
Firstly is the establishment of causality of a disease with an infection. Secondly, measures to prevent the disease in those who are susceptible. Last but not least, genetic manipulation, to be precise in our approach, which can be pursued down the line as has been done with gene editing for hereditary diseases by CRISPR.
In the three disease examples cited, the entry portals to the pathogens after identification can be modified to prevent the entry of the pathogens. This could drastically reduce disease burden in those who are susceptible, preventing chronic morbidity and unnecessary drug treatment and improving quality of life. Gene editing by pinpointing the genes of interest and editing or deleting the concerned loci could be the future of this aspect of chronic disease prevention/treatment.
References
- Matzaraki, V., Kumar, V., Wijmenga, C. et al. The MHC locus and genetic susceptibility to autoimmune and infectious diseases. Genome Biol 18, 76 (2017). https://doi.org/10.1186/s13059-017-1207-1
- O’Connor SM, Taylor CE, Hughes JM. Emerging infectious determinants of chronic diseases. Emerg Infect Dis. 2006 Jul;12(7):1051-7. doi: 10.3201/eid1207.060037. PMID: 16836820; PMCID: PMC3291059.
- Hyoty H, Taylor KW: The role of viruses in human diabetes. Diabetologia 45: 1353–1361, 2002
- Honeyman MC, Stone NL, Harrison LC: T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: potential for mimicry with rotavirus and other environmental agents. Mol Med 4: 231–239, 1998
- Pak CY, Eun HM, McArthur RG, Yoon JW: Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet 2: 1–4, 1988
- Viskari H, Ludvigsson J, Uibo R, Salur L, Marciulionyte D, Hermann R, Soltesz G, Fuchtenbusch M, Ziegler AG, Kondrashova A, Romanov A, Kaplan B, Laron Z, Koskela P, Vesikari T, Huhtala H, Knip M, Hyoty H: Relationship between the incidence of type 1 diabetes and maternal enterovirus antibodies: time trends and geographical variation. Diabetologia 48: 1280–1287, 2005
- Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M: Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47: 225–239, 2004
- .Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P: Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 104: 5115–5120, 2007
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020 Dec 22;76(25):2982-3021. doi: 10.1016/j.jacc.2020.11.010. Erratum in: J Am Coll Cardiol. 2021 Apr 20;77(15):1958-1959. PMID: 33309175; PMCID: PMC7755038.
- Lopez-Candales A, Hernández Burgos PM, Hernandez-Suarez DF, Harris D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J Nat Sci. 2017 Apr;3(4):e341. PMID: 28670620; PMCID: PMC5488800.
- Naga Venkata K Pothineni, Swathi Subramany, Kevin Kuriakose, Lily F Shirazi, Francesco Romeo, Prediman K Shah, Jawahar L Mehta, Infections, atherosclerosis, and coronary heart disease, European Heart Journal, Volume 38, Issue 43, 14 November 2017, Pages 3195–3201, https://doi.org/10.1093/eurheartj/ehx362
- Soldan, S.S., Lieberman, P.M. Epstein–Barr virus and multiple sclerosis. Nat Rev Microbiol 21, 51–64 (2023). https://doi.org/10.1038/s41579-022-00770-5
- Ascherio A, Munger KL. Epstein-barr virus infection and multiple sclerosis: a review. J Neuroimmune Pharmacol. 2010 Sep;5(3):271-7. doi: 10.1007/s11481-010-9201-3. Epub 2010 Apr 6. PMID:
Also, read: The Nabateans: A History Preserved in Rocks

Dr Syed Hunain Riaz is a Physician with expertise and experience in Endocrinology & Metabolism with a passion for eliciting change through the dissemination and application of precise knowledge. He is a space enthusiast, avid reader, blog writer, amateur photographer ( Astro & every day), and gamer.
Writing interests include preventive & lifestyle medicine and psychosocial issues. Dr. Hunain believes in limitless creativity and productivity of the human mind. Nature of consciousness, reality, and patterns in the universe are areas of special interest. He can be reached at syedhunain@gmail.com