“ADHD is a performance disorder. You cannot perform the things you know how to do. It is not a knowledge disorder,” says Dr. Russell Alan Barkley, an American retired clinical neuropsychologist and a clinical professor of psychiatry.
ADHD is a neurological disorder affecting children and their ability to perform function. It is characterized by patterns of inattention or difficulty in paying attention, hyperactivity, and impulsive behavior involving the child’s development. Children with ADHD are often labeled as troublemakers. Inattention is the difficulty of consistently focusing on one task and staying organized. Inattention might include missing deadlines, difficulty following instructions and concentrating on one task, getting distracted, disorganizing tasks, forgetting or losing the necessary things, etc.
Hyperactivity refers to a condition in which a person experiences continuous movement and a high energy level. Impulsivity is when a person loses self-control and does things without thinking. The adults may include the behaviors of making decisions impulsively and without thinking about their long-term results or consequences.
Hyperactivity-impulsivity includes the symptoms of constant motion, talking unnecessarily, interfering with others, speaking loud, aggression, poor time management, anti-social behavior, frustrations, stress, fidgetiness, nervousness, wriggling, restlessness, etc. The adults usually exhibit symptoms related to mood, impulsiveness, and low self-esteem. These symptoms do not appear suddenly, but people continue to experience them, affecting their daily functions.
Commonly, people suffer from such conditions in their everyday lives, too. More often than not, they cannot recognize that they have ADHD because they think that symptoms like missing deadlines, forgetting meetings, or difficulties in everyday tasks are just the challenges of life. However, the condition of ADHD is when the symptoms are very severe and persistent.
Types of ADHD
Usually, the symptoms of ADHD start appearing at the age of three and can continue to adulthood. Then, as they grow, the symptoms of inattention dominate during their school, daily tasks, classrooms, etc., and the adults seem to suffer more from impulsivity, restlessness, aggression, etc. The symptoms can be due to one factor or a combined effect of all conditions.
Based on its symptoms, the American Psychiatric Association (APA) has categorized ADHD into three types.
Predominantly Inactive: In this category, people have symptoms of inattention and inactivity, like difficulty focusing, completing tasks, following guidelines, etc., for at least six months. This type of behavior is more common in girls and often goes undiagnosed because of unawareness and ignoring the symptoms.
Predominantly hyperactive-impulsive: It includes the symptoms of hyperactivity and impulsiveness like fidgetiness, interrupting people, excessive talking, aggression, etc., that have been present for the past six months.

Combined Type: In this, the people display the combined symptoms of both conditions, including inactivity, impulsive behavior, and high activity levels for the last six months. This is the most common type of ADHD in people.
What causes ADHD?
While addressing the causes of ADHD, researchers suggest a combination of genetic, environmental, and developmental factors influencing ADHD. This psychiatric condition is heritable to the next generations and primarily develops the risk in siblings than the other population. Among the environmental factors are brain injuries, malnutrition, exposure to alcohol, smoke, or lead during pregnancy, and developmental problems in the central nervous system, premature birth all contribute to the cause of ADHD.
In general, ADHD is more common in males than females, with a ratio of 5 to 1. In Pakistan, around 2.49% of the population has been diagnosed with ADHD. Sometimes, the symptoms of ADHD coexist with other mental problems like anxiety disorder, mood disorder, depression, stress, and learning disabilities that worsen the condition.
Diagnosis of ADHD
Diagnosis of ADHD is essential. The sooner we recognize the patterns and start working on them, the better it is for the patient’s health. The diagnosis of ADHD seems complicated sometimes because the persons are unaware of the feelings or symptoms they are suffering from, or they can misunderstand the symptoms of other disorders with ADHD.
For diagnosis of ADHD, the symptoms must be present from an early age before 12 years, at least appeared constantly for six months, must be chronic or long-lasting, present in two or more settings like home, school, workplace, etc. and affect the development and normal functions of life. And the symptoms should not be mixed with other disorders like mood disorders, developmental or learning disorders involving the differential diagnosis of the condition.
The diagnosis is provided by primary care providers, mental health professionals, and clinical psychologists. There are no specific laboratory tests to diagnose ADHD; hence, they are done clinically. For this, considering the patient’s history is very important. Then, the symptoms are evaluated clinically based on different scales and involve foreign relations between patients, such as parents and teachers. It can help them get information on the symptoms parents have observed.
Treatment and Medication
The treatment of ADHD patients mainly includes two types of medications for treating the symptoms of the disease: stimulants and non-stimulants, as approved by the Food and Drug Administration (FDA). Neurologists prescribe all these medications, including the American Association of Psychiatric (AAP) and the Center for Disease Control (CDC), for the treatment of patients with different stages of ADHD.
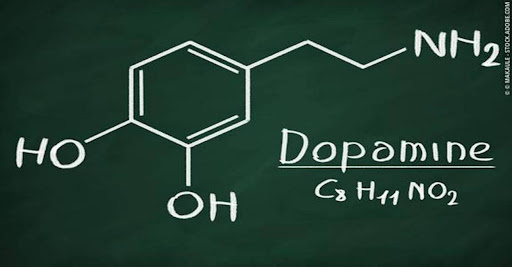
Stimulants are drugs that speed up bodily functions like the brain, heart, and muscles. They can make a person feel more alert, energetic, confident, or happy. These include amphetamines and methylphenidate-based medications, and they both tend to increase the level of dopamine (a neurotransmitter in the brain associated with pleasure, reward, motivation, and motor control) and norepinephrine (a hormone that reacts to stressful situations like increasing your heart rate and blood pressure) in the brain.
Thereby, these stimulate attention, thinking, and focus. Stimulants are the most effective medication in about 70 percent of patients, available in different formulations, and generally considered safe.
According to Dr. Russel Barkly, “These stimulants are a form of genetic therapy; they tend to modify the neurogenetic impact of the disorder in the brain by altering the genes in the brain. But they only work when inside the brain’s bloodstream temporarily.” However, the side effects persist, such as loss of sleep and hunger, alteration in blood pressure, and more dependency on others. These side effects can occur due to misuse or excessive use of medication.
Non-stimulant drugs are medications that can help treat ADHD without affecting the levels of dopamine. They target other brain chemicals, such as norepinephrine, which can also affect attention and behavior. These non-stimulants were approved for treating ADHD in 2003; they also work by enhancing focus and attention and reducing the impulsiveness of patients.
These medications include slow-working antidepressants and alpha agonists, but usually last longer for up to 24 hours. Among antidepressants, the most effective is atomoxetine, which elevates the levels of norepinephrine. Bupropion (increasing dopamine and serotine levels) and TCA (norepinephrine) are also examples of antidepressants. These are mainly recommended for children who cannot tolerate the stimulants or if stimulants are showing side effects or in combined therapy with stimulants.
The other types of non-stimulants include alpha agonists (a class of drugs called sympathomimetics, which mimic the effects of the sympathetic nervous system and are life-saving drugs for heart attacks and treatments for cardiovascular and psychiatric conditions) like clonidine and guanfacine that act on the Central Nervous System and target epinephrine and norepinephrine.
But these also show side effects like disturbing blood pressure, dizziness, and increased weight. The American Association of Psychiatric (AAP) suggests that healthcare providers look at these medications’ benefits and side effects on all patients and then recommend the dosages or treatment.
Psychosocial interventions and Psychotherapy
Psychosocial treatment involves the treatment to help patients with ADHD and their families cope with the symptoms of disorders. One is behavioral therapy, designed to improve a person’s behavior. It includes training the patients and their families, and even in schools, for behavior management and improving normal daily functioning.
Dr. Russel Barkley states, “Cognitive behavioral therapy does a pretty good job in boosting executive functioning in adults on medication.”
The American Psychiatric Association (AAP) has recommended these training and programs as a priority to the medication for children under age 6. For children above 6-12, behavioral therapy is given in combination with medication. Different cognitive-behavioral programs and training are conducted for patients. School behavior interventions are also done.
Several benefits are owing to these behavioral therapies, including:
- Helping the patients to observe, manage, and control their anger and behavior.
- To improve focus and attention by learning to control their thoughts and teaching children social skills to improve their behavior like sharing, asking for help, waiting for their turns, speaking in slow voices, etc.
- Helping the parents, teachers, and family observe and note the children’s behavior and establish the rules and routines accordingly.
- It helps parents deal with and respond to their child’s frustration, managing distractions like TV and noise during their study time and allowing them to organize their books, school bags, toys, etc.
- Behavioral parent management training teaches parents to adopt rewarding behavior with their children and encourages them with positive feedback.
- Furthermore, parents should adopt calm behavior to improve their children’s discipline instead of scolding them, help them break down complicated tasks, and adopt a positive, encouraging, and healthy lifestyle.
- Classroom management interventions help children improve their classroom and peer behavior using less classwork, preferred classroom sitting, and time relaxation during exams.
Natural remedies for ADHD
Besides medication and behavioral therapy, remedies can also help improve the symptoms of ADHD. According to the Centers for Disease Control (CDC), physical activity of at least 60 minutes a day, nutrition and a balanced diet, regular sleep, limiting screen time on TV, phones, etc., adopting outdoor activities, and a positive and calm outlook for life can help ADHD patients relieve their symptoms.
References:
- Magnus, W., Nazir, S., Anilkumar, A. C., & Shaban, K. (2023, August 8). Attention deficit hyperactivity disorder. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK441838/
- Mayo Foundation for Medical Education and Research. (2023, January 25). Adult attention-deficit/hyperactivity disorder (ADHD). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/adult-adhd/symptoms-causes/syc-20350878
- Núñez-Jaramillo, L., Herrera-Solís, A., & Herrera-Morales, W. V. (2021, March 1). ADHD: Reviewing the causes and evaluating solutions. MDPI. https://doi.org/10.3390%2Fjpm11030166
- This is how you treat ADHD based on science, dr Russell Barkley’s part of the 2012 Burnett lecture. YouTube. (2014, September 23). https://youtu.be/_tpB-B8BXk0?si=XTZBZTKtKjwShsPt
- Key Concerns and Strategies for Diagnosing and Treating Adults with ADHD w/ Russell Barkley, Ph.D. YouTube. (2023, April 06). https://youtu.be/W6JBgeFbYCc?si=Qeg_iCW33b7Jmg_E
- U.S. Department of Health and Human Services. (2023, September). Attention-deficit/hyperactivity disorder. National Institute of Mental Health. https://www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd
- Centers for Disease Control and Prevention. (2023, September 27). What is ADHD? Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/adhd/facts.html
- Angel, T. (2023, November 1). Everything you need to know about ADHD. Healthline. https://www.healthline.com/health/adhd
- Nall, R. (2023, November 16). Attention-deficit/hyperactivity disorder. National Institute of Mental Health. https://www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd
Similar Posts: Let’s talk about Mental Health in Pakistan